OGYÉI’s integration into the new authority
As of 1 August 2023, the National Institute of Pharmacy and Nutrition (OGYÉI) shall be merged into the National Public Health Center. The new authority will be called the National Center for Public Health and Pharmacy (NNGYK).
The new authority will be headed by the Chief Medical Officer, and in terms of administrative hierarchy, the NNGYK will be under the supervision of the Minister of the Interior, acting in the capacity of Minister responsible for Health.
The NNGYK may act as OGYÉI’s general legal successor or may join any ongoing official or judicial proceedings initiated by the OGYÉI or in which the OGYÉI participates as a party. In the future, the NNGYK will carry out OGYÉI’s competences, including, in particular, authority inspections related to medicinal products, in vitro diagnostics and medical devices and cosmetic products.
Retrospective: statistics of 13 years of OGYÉI case law
On the occasion of the OGYÉI’s termination as an independent authority, we prepared an overview on the statistics of the OGYÉI caselaw of the past 13 years. The reason for selecting this was that summaries of the authority’s decisions have been published on the OGYÉI’s website going back 13 years.
1. Inspection Frequency
Firstly, we present the frequency of authority inspections, on the basis of which an authority’s general activity and the probability of enforcement can be measured. In the past 13 years, OGYÉI, as the authority supervising medicinal product promotion, initiated 54 procedures, while only 2 official decisions were published on the authority’s website regarding supervision of wholesale trade in medicinal products.
As illustration, we prepared a graph on the authority inspections related to medicinal product promotions, since it is the larger data set. The graph shows the number of inspections initiated each year over the last 5 years, and the average over the entire 13 years period as a benchmark. In view of the fact that OGYÉI merges into the NNGYK during the calendar year, we also prepared a projection for the rest of 2023. The projection presumes that OGYÉI would initiate proportionally the same number of cases in the remaining 5 months of the year, and is for information purposes only.
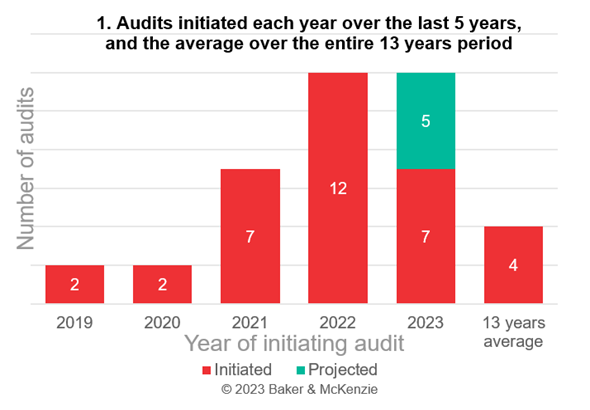
Based on the above graph, it can be clearly stated that the OGYÉI’s activity as the supervisory authority over medicinal product promotion has recently shown an upward trend: it should be noted that in 2022, three times as many proceedings were initiated as the average of 13 years, and that more than half of all proceedings were initiated in the last 5 years.
When assessing the likelihood of an authority inspection, it is also advisable to take into account the frequency with which OGYÉI inspects companies that have already been audited once. Based on the 54 cases published so far, it can be established that in at least 9 cases, the OGYÉI inspected the same company or another member of the same company group, i.e. nearly one fifth of the audits were initiated against the same company or group. This means that pharmaceutical companies cannot reasonably expect that once they have passed OGYÉI’s inspection, they will not be audited again.
2. Amount of administrative fines imposed
Following the presentation of the frequency of case openings, we examine the amount of administrative fines imposed by OGYÉI. On the basis of prior legal practice, we can deduct rough estimates as to the amount of fines that an audited company can expect.
The highest administrative fines imposed by OGYÉI have been related to the 2 cases regarding supervision of wholesale trade in medicinal products: in one case, a fine of HUF 500,000,000 (approx. EUR 1,300,000), which is the maximum under the relevant Hungarian law, and in the other case, HUF 98,000,000 (approx. EUR 255,000) was imposed. The higher fines for wholesale related infringements are presumably justified by the high values protected by the respective legal provisions (risks to public health and drug safety), that merit greater protection, thus more severe consequences. In view of the fact that no further decisions are available on the wholesale trade of medicinal products, we present the data on OGYÉI’s supervision practice over medicinal product promotion.
The highest administrative fine imposed by OGYÉI relative to medicinal product promotion was HUF 50,000,000 (approx. EUR 130,000), which was imposed in 2011. However, the past 13 years of OGYÉI practice have been characterised by the imposition of fines approaching, but never quite reaching this amount.
On average, OGYÉI imposed an administrative fine of approximately HUF 16,000,000 (approx. EUR 42,000). The median fine was slightly lower, rounded to HUF 14,000,000 (approx. EUR 36,000). Based on a total of the 54 published decisions, it can also be concluded that no company received a fine of less than HUF 1,500,000 (approx. EUR 4,000). As a result, the latter three amounts should be considered as highly likely, and the last one specifically the very least that an audited company should expect as a possible outcome.
The graph below shows the total amount of fines imposed by OGYÉI as a result of official inspections launched in each year, based on the data of the last 5 years and compared to the average of the publicly available data over the last 13 years. The projection presumes that OGYÉI would impose proportionally the same amount of administrative fines in the remaining 5 months of the year, and is for information purposes only.
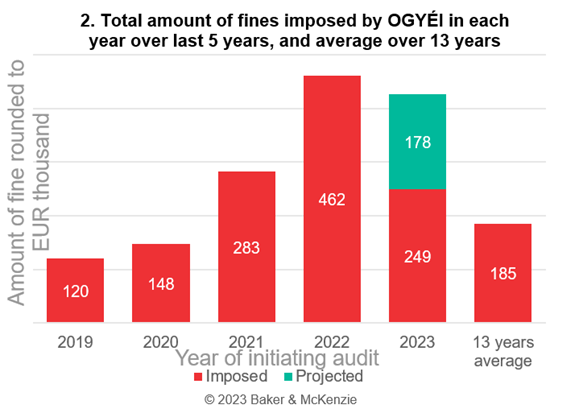
If we compare the first and second graphs, it can be concluded that an increase in the number of proceedings does not necessarily mean that the total amount of fines imposed in cases initiated in a given year will also increase proportionately. This is presumably due to the fact that in the years with fewer cases, OGYÉI conducted investigations of greater scope and significance, which could have occupied the authority’s capacities, but ultimately resulted in higher fines on average.
Based on the above data, the past 13 years of OGYÉI as the independent pharmaceutical authority show the image of an active authority, especially if we take into account that, unlike authorities in other areas of law, OGYÉI specialises exclusively in one industry, and that the authority’s activity has further increased based on the most recent case numbers.
Our thoughts
We expect that the integration of OGYÉI into the new authority, the NNGYK, will not result in any significant changes in terms of the established trend. Further, it is most likely that, within the NNGYK, the pharma organisational unit will continue authority inspections with unwavering activity and relative autonomy to the extent possible. Therefore, the change could be considered more of a formality. In any case, the above statistics of the OGYÉI can serve as a basis for comparison when examining the pharma authority practices of the NNGYK.




