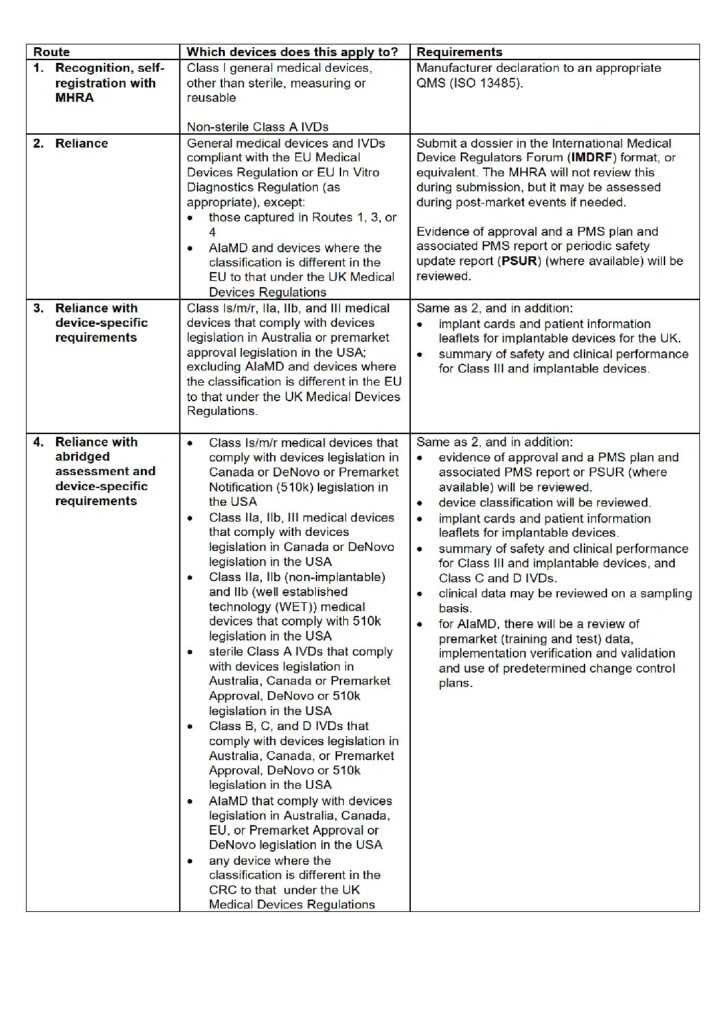
The UK’s MHRA has published a statement of policy intent for recognition of international regulators’ approvals of medical devices in Great Britain (GB). If your organisation’s medical device is authorised in any of Australia, Canada, the EU, or USA (together, Comparable Regulator Countries or CRCs), you may be able to leverage this to gain fast-tracked access to the GB market. This means that manufacturers may be able to side-step the full UK authorisation process for an abridged version or a simpler submission requirement. However, the statement is not a magic bullet: it does not apply to Northern Ireland (where EU law continues to apply); there are a significant number of devices which are excluded from this process; and the statement does not represent law yet, although it should become effective once the future UK Medical Device Regulations enter into force (planned for 2025).
How will international recognition work?
The requirements for this proposed access route depend on the classification of the relevant device under UK regulations. If successful, a manufacturer will obtain a certificate of international recognition (but not a UKCA mark) and will need to comply with conditions around requirements in the relevant CRC and regarding access to the GB market. These include requirements to have a UK Responsible Person, UDI requirements, English language labelling and packaging, complying with GB requirements for electronics compatibility, and compliance with the new post-market surveillance (PMS) requirements in the future UK Medical Devices Regulations.
If a medical device is eligible to utilise this process, market access will be in accordance either with:
- the validity of the supplied certificate from the CRC (and must be re-certified when their original certificate expires).
- where the CRC in question allows indefinite market access (such as in the USA), the QMS certificate.
The MHRA will continue to recognise CE certification and approvals completed by the EU until 30 June 2030, at the latest. Manufacturers will still have the option to use the UKCA marking instead to place devices on the GB market.
What’s excluded from the international recognition procedure?
Excluded devices include:
- custom-made devices
- Software as a Medical Device (SaMD) (including Artificial Intelligence as a Medical Device (AIaMD)) products that do not satisfy the MHRA’s intended purpose guidelines
- SaMD (including AIaMD) products approved via a route which relies on equivalence to a predicate (US 510(k))
- products granted market access in the CRC via a recognition route
- Class IIb (non-well established technology (WET)) implantable and III medical devices approved via a route which relies on equivalence to a predicate (US 510(k))
- companion diagnostics approved via a route which relies on equivalence to a predicate (US 510(k))
- companion diagnostics and combination products containing medicines that are not licensed in the UK
- products excluded from the scope of UK MDR 2002, listed in Regulation 3
What are the proposed access routes?