Baker McKenzie has recently conducted a survey across over 100 jurisdictions, asking our Healthcare & Life Sciences global network of specialists to compare the degree of flexibility clinical trial sponsors have to negotiate with sites in clinical trial agreements.
We have received input from our healthcare regulatory experts in 105 jurisdictions and are therefore able to build a global picture of how the flexibility afforded to sponsors varies from market to market.
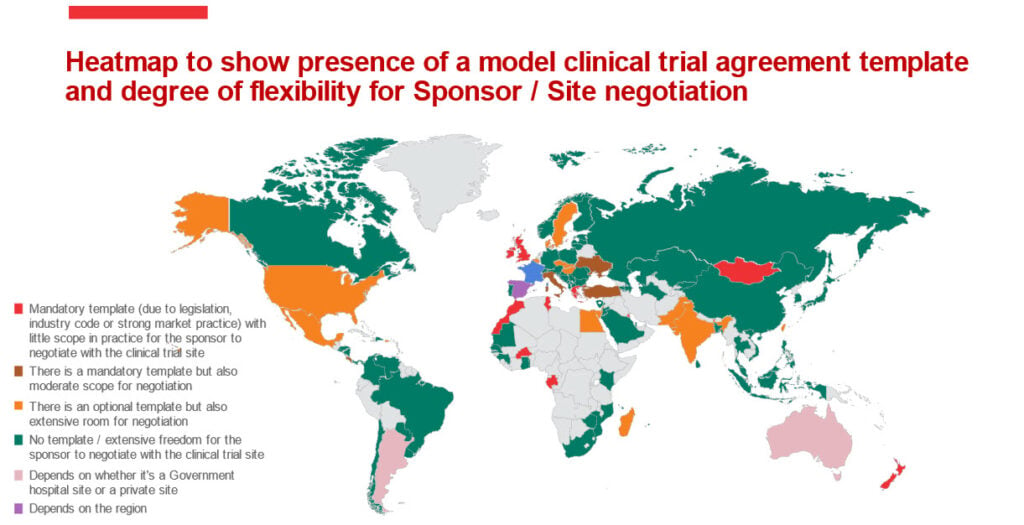
The results are fascinating and the snapshot results can be seen in the heatmap below, highlighting markets where, like in the UK, there is a model clinical trial agreement template from which in practice there can be little or no deviation, in stark contrast to markets such as Canada where there is a much greater flexibility for sponsors to negotiate with sites.
Across the landscape it is clear that the degree of flexibility for the sponsor to negotiate with the clinical trial site on a clinical trial agreement is jurisdiction-specific.
We have identified markets in the different categories across the spectrum as follows:
- Jurisdictions with no template available from the regulator / with extensive freedom for the sponsor to negotiate with the clinical trial site include: Austria, Brazil, Bulgaria, Canada, Chile, China, Finland, Japan, Kazakhstan, Russia and Saudi Arabia.
- Jurisdictions where there is an optional template available from the regulator but allowing for extensive negotiation include: Belgium, Czech Republic, Denmark, Egypt, Hungary, India, Israel, Sweden and the US.
- Jurisdictions where there is a mandatory template with moderate scope for negotiation include: Costa Rice, Italy, Turkey and Ukraine.
- Jurisdictions where there is a mandatory template with minimal scope of flexibility in practice (due to legislation, industry code or strong market practice) include: France, Greece, Ireland, Morocco, New Zealand and the UK.
- Finally, there are jurisdictions where the degree of flexibility for the sponsor to negotiate with the clinical trial site on the clinical trial agreement would depend on whether it concerns a Government hospital site or a private site (e.g. Argentina and Australia) or depend upon the region in the country (e.g. Spain).
For further information on this survey please contact Julia Gillert, Els Janssens, Jaspreet Takhar, Magda Tovar, Elina Angeloudi or Ariel Leung.








